Lumigan + Applicators
Lumigan + Applicators
- In our pharmacy, you can buy Lumigan + applicators without a prescription, with delivery in 5–14 days throughout the United Kingdom. Discreet and anonymous packaging.
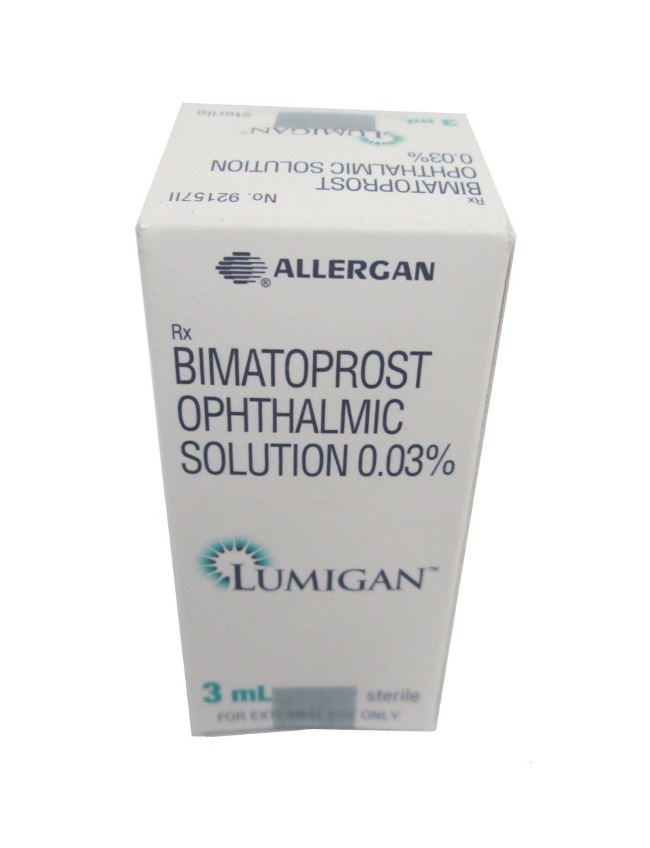
- Lumigan is used for the treatment of elevated intraocular pressure in open-angle glaucoma and ocular hypertension. Its active ingredient, bimatoprost, works by increasing the outflow of aqueous fluid from the eye, thereby reducing intraocular pressure.
- The usual dose of Lumigan is 1 drop in the affected eye(s) once daily in the evening.
- The form of administration is an ophthalmic solution, typically in a dropper bottle.
- The effect of the medication begins within 4–8 hours.
- The duration of action is approximately 24 hours.
- Do not consume alcohol.
- The most common side effect is conjunctival hyperemia (red eyes).
- Would you like to try Lumigan + applicators without a prescription?
Lumigan + Applicators
Basic Lumigan + Applicators Information
- INN (International Nonproprietary Name): Bimatoprost
- Brand Names Available in United Kingdom: Lumigan
- ATC Code: S01EE03
- Forms & Dosages: Ophthalmic solution (0.01% - 2.5 mL, 3 mL, 5 mL bottles)
- Manufacturers in United Kingdom: Allergan (an AbbVie company)
- Registration Status in United Kingdom: Prescription only
- OTC / Rx Classification: Prescription only (Rx)
Everyday Use & Best Practices
When it comes to Lumigan, proper usage is vital for achieving the desired results in managing eye pressure. One key aspect is the timing of doses. The recommended regimen for Lumigan is one drop in the affected eye(s) once daily, ideally in the evening. This timing is crucial as using it consistently at the same time each day helps maintain stable intraocular pressure. Variability in dosing can lead to fluctuations that may impact the treatment's efficacy.
Taking With or Without Meals
It’s often a concern whether to take Lumigan with food. Generally, it can be taken with or without meals. However, patients might find that having it with lighter meals—such as toast or cereal—can be less irritating. Given common UK diet habits, such as enjoying a hearty breakfast or traditional fish and chips in the evening, it’s important to note that very greasy or spicy foods might lead to gastrointestinal discomfort, potentially affecting how a patient feels post-application.
Safety Priorities
Awareness of safety is paramount when using Lumigan. Certain populations or conditions should avoid this medication. The UK Medicines and Healthcare products Regulatory Agency (MHRA) indicates that patients with hypersensitivity to bimatoprost or any ingredients present in Lumigan should not use it. Additionally, it is not recommended for children under three years of age.
Activities To Limit
After applying Lumigan, it’s essential to be mindful of activities that require clear vision. Driving or operating machinery should be limited until vision is stable. Should a patient experience any blurriness or visual disturbances post-application, it’s advisable to wait until clarity returns before engaging in such tasks, ensuring safety for both the individual and others.
Dosage & Adjustments
According to NHS guidelines, the standard regimen for managing elevated intraocular pressure due to glaucoma with Lumigan is one drop in the affected eye(s) once daily in the evening. It is important for patients to adhere strictly to this scheduling, as inconsistencies can undermine treatment effectiveness.
Special Cases
Caution should be exercised when prescribing Lumigan to certain groups, such as the elderly or patients with liver or kidney impairments. For older patients, regular monitoring is advised, although no specific dosage adjustments are typically necessary. Those with severe liver impairment may require closer supervision, as there is limited data on the need for dosage alterations in this demographic.
User Testimonials
Positive experiences among UK patients highlight the effectiveness of Lumigan. Many report impressive results in managing their glaucoma and appreciate the ease of use. For example, comments on platforms like Patient.info note that users have found their intraocular pressure significantly reduced and have expressed satisfaction with the overall management of their condition.
Common Challenges
However, it’s also important to acknowledge the challenges some patients face, which have been discussed on forums like NHS and Patient.info. Common concerns include potential side effects like conjunctival hyperemia or changes in eyelash growth. Understanding these experiences can help new users prepare for possible outcomes and discuss any issues with their healthcare providers.
Buying Guide
When it comes to sourcing Lumigan, major pharmacies across the UK, including Boots, LloydsPharmacy, and Superdrug, provide options for purchasing this medication. Customers can also explore online services offered by these pharmacies for added convenience.
Price Comparison
Understanding the cost of Lumigan is crucial. In the UK, the NHS prescription charge typically applies, though this may vary across Scotland, Wales, and Northern Ireland where different pricing regulations exist. It's always beneficial to check local guidelines and the specific pharmacy policies to get the best pricing.
What’s Inside & How It Works
Ingredients overview
Lumigan contains bimatoprost as its active ingredient, a powerful prostaglandin analogue. This ingredient is primarily used to lower intraocular pressure (IOP) in individuals with glaucoma or ocular hypertension. Bimatoprost works by increasing the outflow of fluid from the eyes, helping to prevent potential damage to the optic nerve that can occur with elevated pressure. Alongside bimatoprost, the formulation includes excipients like benzalkonium chloride, which acts as a preservative to enhance the product's shelf life.
Mechanism basics explained simply
Understanding how bimatoprost works is crucial for anyone dealing with high eye pressure. It primarily functions by mimicking the effects of natural prostaglandins, compounds your body produces. Bimatoprost enhances fluid drainage from the eye, which effectively reduces IOP. Think of it like opening a tap to let excess water flow out—this flushing action prevents pressure build-up that could harm vision in the long run.
Main Indications
Approved uses
According to MHRA guidelines, Lumigan is approved for treating elevated intraocular pressure associated with open-angle glaucoma and ocular hypertension. It is prescribed for adults who require pharmacological intervention to lower their eye pressure, and patients are advised to use it once daily in the evening for optimal effect. Regular monitoring is essential to ensure the treatment remains effective.
Off-label uses in UK clinics
In addition to its approved therapeutic uses, Lumigan has gained popularity for off-label applications, particularly in cosmetic medicine for eyelash growth. Branded as Latisse in some regions, this usage capitalises on bimatoprost's ability to enhance eyelash length and thickness. Patients using Lumigan for this purpose should do so with sterile applicators to minimise any risk of unintended hair growth in other areas.
Interaction Warnings
Food interactions
Dietary habits can impact treatment efficacy. While no significant food interactions have been highlighted, moderate alcohol consumption might lead to dehydration and increase dry eye symptoms. Caffeinated drinks such as tea or coffee can also contribute to dry eyes and discomfort while using Lumigan. It's advisable to maintain a balanced diet and consult a healthcare provider for tailored advice.
Drug conflicts
Consulting with healthcare providers regarding potential drug interactions is crucial. Known conflicts include certain antihypertensives and systemic medications that might influence eye pressure. MHRA Yellow Card reports have noted interactions that could lead to adverse effects or reduced therapeutic efficacy. Patients need to disclose all medications they are currently taking to their doctor to ensure safe use of Lumigan.
Latest Evidence & Insights
Recent studies in the UK and EU have reinforced Lumigan's efficacy and safety profile from 2022 to 2025. Research indicates that patients using Lumigan demonstrate significant reductions in IOP compared to those on alternative treatments. Long-term studies have further shown that side effects are generally manageable and often mild. Upsurge in its off-label cosmetic use has prompted additional investigations into safety, confirming minimal risks with proper application methods using applicators.
Alternative Choices
For those seeking alternatives on the NHS for treating glaucoma, several options are available, including:
- Xalatan (Latanoprost): Another prostaglandin analogue.
- Travatan (Travoprost): Similar action with different formulation.
- Taflotan (Tafluprost): Offers similar benefits.
- Ganfort: Combination of bimatoprost and timolol for enhanced pressure reduction.
While each medication carries its own advantages and drawbacks, overall efficacy varies based on individual responses. It's essential to discuss with a healthcare professional to weigh the pros and cons, ensuring the chosen treatment aligns with patient needs while considering side effects. Regular monitoring and adherence to treatment plans are vital to managing glaucoma effectively.
Regulation Snapshot
Lumigan, known generically as bimatoprost, holds a prescription-only status in the UK, tightly regulated to ensure patient safety and efficacy. The Medicines and Healthcare products Regulatory Agency (MHRA) oversees its approval, ensuring that the product meets stringent safety and quality standards before it reaches patients. This involves close monitoring of adverse effects and a continual assessment of its therapeutic benefits.
Within the National Health Service (NHS) framework, Lumigan is prescribed primarily for managing elevated intraocular pressure in patients with open-angle glaucoma and ocular hypertension. Patients are typically instructed to use one drop in the affected eye(s) once daily in the evening to maintain efficacy. NHS guidelines stress the importance of adhering to the prescribed regimen, as more frequent dosing may diminish the overall effectiveness of the treatment.
FAQ Section
Common UK patient questions
Many patients may have queries about Lumigan, its use, and potential side effects. Here are some common questions answered:
- What is Lumigan used for? Lumigan is primarily prescribed to reduce elevated intraocular pressure in patients with glaucoma or ocular hypertension.
- How should I use Lumigan? The typical regimen involves applying one drop in the affected eye(s) once daily in the evening.
- Are there any side effects I should be aware of? Common side effects include red eyes, eye irritation, and possible eyelash growth. Consult your healthcare provider for a complete list.
- Is Lumigan suitable for everyone? It is not recommended for children under three or individuals with hypersensitivity to its ingredients.
- What if I miss a dose? If a dose is missed, it should be applied as soon as remembered, except when close to the next scheduled dose—do not double dose.
Guidelines for Proper Use
UK pharmacist counselling style
When a patient is prescribed Lumigan, pharmacists typically adopt a straightforward and supportive counselling approach. Key points they cover include:
- Importance of adherence to the once-daily dosing regimen to maximise effectiveness.
- How to administer the drops correctly—avoid touching the dropper to any surface.
- Discussion of potential side effects and reassurance regarding common reactions.
- Clear instructions on storage, ensuring Lumigan is kept at the appropriate temperature and protected from light.
This style helps empower patients, enhancing their understanding of the therapy and promoting better adherence to treatment.
NHS patient support advice
The NHS offers various resources to support patients using Lumigan, fostering engagement and adherence. Patients have access to:
- Consultation with healthcare professionals for advice and any concerns.
- Informative leaflets and guides about Lumigan and its benefits.
- Access to eye health checks and regular monitoring to assess treatment efficacy.
By utilizing these resources, patients can stay informed, ensuring they make educated decisions about their eye health and effectively manage their condition.
| City | Region | Delivery Time |
|---|---|---|
| London | Greater London | 5–7 days |
| Birmingham | West Midlands | 5–7 days |
| Manchester | Greater Manchester | 5–7 days |
| Glasgow | Scotland | 5–7 days |
| Leeds | West Yorkshire | 5–7 days |
| Cardiff | Wales | 5–7 days |
| Bristol | South West England | 5–7 days |
| Sheffield | South Yorkshire | 5–7 days |
| Edinburgh | Scotland | 5–7 days |
| Newcastle upon Tyne | North East England | 5–7 days |
| Nottingham | East Midlands | 5–9 days |
| Coventry | West Midlands | 5–9 days |
| Plymouth | South West England | 5–9 days |
| Stoke-on-Trent | West Midlands | 5–9 days |