Myfenax
Myfenax
- In our pharmacy, you can buy myfenax without a prescription, with delivery in 5–14 days throughout the United Kingdom. Discreet and anonymous packaging.
- Myfenax is intended for the prophylaxis of organ rejection in allogeneic renal, cardiac, and hepatic transplants. The drug works as an immunosuppressant by inhibiting purine synthesis.
- The usual dosage of myfenax for renal transplant is 1 g twice daily; for cardiac and hepatic transplants, it is 1.5 g twice daily.
- The form of administration is an oral tablet or an intravenous (IV) formulation.
- The effect of the medication begins within 1–2 hours after administration.
- The duration of action is approximately 12 hours.
- Do not consume alcohol.
- The most common side effect is gastrointestinal issues, including diarrhea and nausea.
- Would you like to try myfenax without a prescription?
Myfenax
Basic Myfenax Information
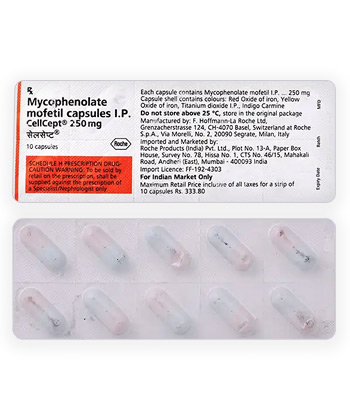
- INN (International Nonproprietary Name): Mycophenolate mofetil
- Brand names available in United Kingdom: CellCept, Myfenax, Mycophenolate mofetil Accord
- ATC Code: L04AA06
- Forms & dosages: Tablets (500 mg), Capsules (250 mg), Oral Suspension (200 mg/ml), IV (500 mg/vial)
- Manufacturers in United Kingdom: Roche, Accord Healthcare, Sandoz
- Registration status in United Kingdom: Prescription-only medication (Rx)
- OTC / Rx classification: Prescription-only (Rx)
Everyday Use & Best Practices
Morning Vs Evening Dosing
Adhering to a consistent dosing schedule is vital for managing myfenax, or mycophenolate mofetil. It’s generally advised to take this medication twice daily, ideally in the morning and evening. This routine helps in maintaining stable drug levels in the body, contributing to a lower risk of organ rejection following transplantation. While some patients prefer evening doses to align with their other medications or nightly habits, it’s important to tailor timing based on personal experiences. If gastrointestinal discomfort arises, adjusting the timing to coincide with meals might prove beneficial. Always approach a healthcare provider to discuss your specific dosing schedule to ensure maximum efficacy and comfort.
Taking With Or Without Meals
Myfenax offers flexibility in consumption, as it can be taken with or without food. Yet, many patients find it easier to ingest the medication alongside meals to reduce gastrointestinal side effects, which may include nausea or abdominal pain. In the UK, where meals are substantial and often routine, timing doses with breakfast or dinner can enhance tolerance. Maintaining hydration and a balanced diet is also crucial for patients on immunosuppressants. It's also essential to be conscious of dietary interactions—administrating myfenax right before or after high-fat meals might affect its absorption. If any uncertainties about dietary considerations arise, consulting with a pharmacist is always recommended.
Safety Priorities
Who Should Avoid It
Certain groups must refrain from using myfenax due to serious safety concerns. The MHRA outlines that individuals with hypersensitivity to mycophenolate mofetil, active severe infections, or previous gastrointestinal perforations should avoid this medication. Moreover, pregnant or breastfeeding women are cautioned against it due to the associated teratogenic risks affecting infants. Elderly patients should exercise caution, given their heightened susceptibility to infections and other side effects. Regular monitoring of both kidney and liver functions, as well as comprehensive blood counts, is vital for all patients, especially those in vulnerable demographics.
Activities To Limit
Patients on myfenax should be attentive to potential side effects that may impair everyday activities. Drowsiness and dizziness can significantly affect one’s ability to drive or operate machinery, particularly when commencing treatment or adjusting dosages. It’s prudent to evaluate personal tolerance and speak with healthcare professionals before partaking in such activities. Those in workplaces where safety is paramount—like construction—should discuss their medication with employers to ensure adequate safety measures are in place. If side effects are experienced, considering alternative transportation methods could be a wise choice.
Dosage & Adjustments
General Regimen
For adults after organ transplantation, the standard starting regimen for myfenax often begins at 1 g taken twice daily for renal transplant recipients. In contrast, those receiving cardiac or hepatic transplants may start with 1.5 g twice daily. NHS guidelines advocate for close observation concerning drug responses and side effects, with adjustments made under medical supervision as needed. In cases of renal or hepatic impairments, dosage reductions may be necessary. Patients over the age of 65 should also be monitored more closely. Dosing for paediatric patients is determined according to body surface area, typically around 600 mg/m² per dose. Ongoing follow-ups with healthcare providers ensure that personalisation of care is maintained effectively.
Special Cases
For elderly individuals and those with comorbidities such as renal or hepatic impairment, myfenax dosing requires special attention. Older adults are often at an elevated risk of experiencing infections and other side effects, necessitating careful monitoring. While mild renal impairment might not typically require dose changes, professionals should be cautious when confronting severe renal issues, potentially opting for alternative treatments. Patients with existing health conditions should communicate consistently with their healthcare team to assess for possible interactions with current medications. Tailored monitoring schedules are crucial to ensure the safe and effective management of immunosuppression among these sensitive patient populations.
User Testimonials
Positive Reports From UK Patients
Experiences with myfenax among UK patients vary, with many expressing satisfaction regarding its effectiveness in preventing organ rejection post-transplant. Users often convey a sense of restored health and the ability to return to an active lifestyle. Success stories highlighted on platforms like Patient.info reflect positive outcomes for kidney and liver transplant recipients, showcasing the gradual transition back to everyday life. Taking myfenax with meals has been noted to alleviate side effects, leading to enhanced tolerability. The communal support shared among users serves as an essential encouragement for newcomers to adhere to their treatment regimens.
Common Challenges
Despite being generally well-received, myfenax users often cite common challenges that can be discouraging. Frequent reports of gastrointestinal discomfort—such as diarrhoea and nausea—are prevalent in discussions on NHS forums and Patient.info. This discomfort may sometimes lead to missed doses, jeopardising transplantation success rates. Additionally, the complexities of managing this medication alongside other prescriptions can overwhelm new transplant recipients. Patients frequently seek support from fellow users, focusing on shared coping strategies for managing side effects and emphasizing the critical need for open communication with their healthcare teams. Sharing experiences builds resilience, fostering a supportive and empowered community around myfenax use.
Buying Guide
Pharmacy sources (Boots, LloydsPharmacy, Superdrug)
In the UK, myfenax can be sourced from well-known pharmacy chains like Boots, LloydsPharmacy, and Superdrug. To obtain this medication, patients typically need a prescription from their GP or a specialist. Once the prescription is in hand, it can be taken to these pharmacies for dispensing. Many of these retailers also provide online services that facilitate ordering medications from the comfort of home. Availability may differ from one pharmacy to another, so it's wise to check local stock levels. Notably, larger pharmacy chains may offer prescription delivery services, enhancing access for patients who find it challenging to visit a pharmacy physically.
Price comparison (NHS prescription charge vs private)
Myfenax is available as an NHS prescription medication, offering many eligible individuals the chance to access it free of charge. This is especially true in Scotland, Wales, and Northern Ireland. However, in England, patients without exemption will need to pay the NHS prescription charge, which varies based on dosage and frequency. For those without NHS coverage, prices can differ significantly across pharmacies, and private prescriptions are typically more costly. Some online pharmacies offer competitive rates, but it's crucial to ensure that they are registered with the MHRA and licensed. Considering the importance of myfenax for health, it's essential to balance cost and necessity, as the benefits generally surpass financial concerns.
What’s Inside & How It Works
Ingredients overview
At the heart of myfenax is mycophenolate mofetil, a selective immunosuppressant crucial for preventing organ rejection following transplants. Available in various forms, including 250 mg capsules and 500 mg tablets, it allows flexibility in dosing tailored to individual patient needs. For younger patients, an oral suspension version is available, making administration simpler. The formulation also includes excipients that assist in the effective delivery of the active ingredient. Those with allergies should thoroughly review the product information leaflet or consult a pharmacist for detailed information about all ingredients.
Mechanism basics explained simply
Myfenax exerts its effects by inhibiting the enzyme inosine monophosphate dehydrogenase (IMPDH), which plays a vital role in the purine synthesis pathway in lymphocytes. By blocking this enzyme, the drug significantly reduces the proliferation of B and T lymphocytes, the immune system's key players. This suppression is essential in preventing the body from rejecting transplanted organs, moderating the immune response to foreign tissues. Understanding this mechanism highlights why consistent adherence to myfenax therapy is crucial for transplant patients. Discontinuing treatment could lead to increased risks of organ rejection, making ongoing discussions with healthcare providers about the drug’s mechanism vital for patient empowerment.
Main Indications
Approved uses (MHRA listing)
Myfenax is primarily approved for the prophylaxis of organ rejection in patients who have undergone allogeneic transplants—these include kidney, heart, or liver transplants. The MHRA supports its usage for individuals aged three months and older, both in adults and children. Clinical studies have documented its efficacy in preventing acute rejection episodes, leading to improved transplant outcomes. Beyond its primary indications, myfenax is sometimes prescribed off-label for autoimmune disorders such as lupus nephritis when standard treatments prove ineffective. Always consult a healthcare provider to explore its suitability and considerations for specific cases.
Off-label uses in UK clinics
In addition to its recognised uses, myfenax is frequently prescribed off-label for autoimmune conditions, including lupus nephritis and rheumatoid arthritis in UK clinics. Its immunosuppressive properties can be beneficial for patients who have not experienced relief from standard therapies. Research and case studies underline its positive impact on managing such conditions and enhancing patients' quality of life. However, while off-label use may offer significant relief, understanding the potential risks is crucial. Physicians typically monitor off-label patients closely to adapt treatment and minimise side effects. Ensure open communication regarding the goals of treatment and the evidence base when considering myfenax for off-label uses.
Interaction Warnings
Food interactions (alcohol, tea/coffee)
Patients on myfenax should be cautious about certain food interactions that may influence the medication’s effectiveness. Limiting alcohol consumption is advisable, as it can escalate side effects like gastrointestinal discomfort and also amplify the risk of liver toxicity, particularly in those with existing liver conditions. When it comes to caffeinated beverages such as tea and coffee, while moderate consumption is generally deemed safe, excessive caffeine intake might affect drug absorption. It's recommended to maintain moderation and to discuss personalised dietary guidance with a healthcare provider to optimise the therapeutic benefits of myfenax while ensuring overall health.
Drug conflicts (MHRA Yellow Card reports)
Myfenax can interact with several medications, leading to serious complications. Antacids and certain antibiotics are known to potentially interfere with its absorption, thereby altering concentration levels within the bloodstream. The MHRA operates a Yellow Card system that invites users to report any adverse reactions or unexpected drug interactions, thereby enhancing patient safety and monitoring. Patients should provide their healthcare providers with a comprehensive list of all medications, including over-the-counter products, to evaluate compatibility. Regular check-ins with pharmacists or doctors help mitigate risks associated with polypharmacy, ensuring safer management of treatments.
Latest Evidence & Insights
Recent studies from UK and EU clinical trials have assessed the long-term efficacy and safety of myfenax in both transplant patients and those with autoimmune disorders. Emerging evidence suggests improved graft survival rates and reduced incidence of acute rejection in patients controlled with myfenax compared to traditional therapies. Studies also indicate that it may offer better gastrointestinal tolerability compared to alternative immunosuppressants, particularly in the elderly population. Continuous monitoring is essential for compiling data on long-term outcomes, helping refine treatment protocols and improve patient counselling. Collectively, these findings reinforce the important role of myfenax in modern transplantation and autoimmune management strategies.
Alternative Choices
NHS prescribing alternatives with pros/cons checklist
NHS prescribers may consider other immunosuppressants as alternatives to myfenax, including tacrolimus, azathioprine, and sirolimus. Each has unique advantages and disadvantages. For instance:
- Tacrolimus: Higher risk of nephrotoxicity but more effective in preventing rejection.
- Azathioprine: Older, potentially fewer side effects, but longer onset time.
- Sirolimus: Supports renal function but comes with its own side effects.
A detailed checklist can help weigh these factors against patient needs, including monitoring protocols, potential interactions, and cost implications. Individual circumstances should drive the selection process, necessitating close clinician-patient collaboration to optimise treatment goals.
Regulation Snapshot
MHRA approval & NHS prescribing framework
Myfenax is regulated by the MHRA, ensuring it meets rigorous safety and efficacy standards prior to market approval. The NHS provides clear guidelines for prescribing myfenax, defining conditions for use in line with clinical evidence. Regular audits and patient reviews are integral to the NHS framework, enhancing prescription practices and allowing adjustments based on emerging data. Patients are encouraged to engage actively in discussions regarding their treatment plans and understand their rights within the NHS prescription system, ensuring an informed approach to medication management.
FAQ Section
1. What side effects should I monitor while on myfenax?
Common side effects include gastrointestinal issues, fatigue, and increased infection risk. It’s essential to report severe reactions to your healthcare provider immediately.
2. Can I take myfenax while pregnant?
No, myfenax is not recommended during pregnancy due to teratogenic effects. Discuss alternative treatments with your doctor if you are pregnant or planning to conceive.
3. How should I store myfenax?
Store at room temperature away from moisture and heat. Ensure oral suspension is refrigerated and used within 60 days after reconstitution.
Guidelines for Proper Use
UK pharmacist counselling style
Pharmacists are essential resources for patients taking myfenax. They play a pivotal role in providing counselling on adherence, managing side effects, and advising on potential medication interactions. Patients should feel empowered to discuss their medication regimen transparently and report any adverse effects promptly. Additionally, pharmacists can assist in creating tailored medication schedules that align with the patient's lifestyle. Furthermore, they can educate patients about the importance of consistent follow-up appointments, necessary blood tests, and overall health management strategies while on immunosuppressants.
NHS patient support advice
NHS resources offer valuable support for patients on myfenax, ensuring access to information on the medication, potential side effects, and monitoring requirements. Patients are advised to engage actively with their healthcare providers and report any side effects. Furthermore, NHS helplines and online resources provide additional guidance. Support groups and networks may offer shared experiences to help navigate treatment journeys, enhancing adherence and confidence in managing health post-transplant. Access to mental health resources is also encouraged, acknowledging the psychological impact of chronic medication and the need for holistic care.
Delivery Information
| City | Region | Delivery Time |
|---|---|---|
| London | England | 5–7 days |
| Birmingham | England | 5–7 days |
| Manchester | England | 5–7 days |
| Glasgow | Scotland | 5–7 days |
| Liverpool | England | 5–7 days |
| Edinburgh | Scotland | 5–7 days |
| Bristol | England | 5–7 days |
| Cardiff | Wales | 5–7 days |
| Sheffield | England | 5–9 days |
| Leicester | England | 5–9 days |
| Newcastle | England | 5–9 days |
| Nottingham | England | 5–9 days |
| Coventry | England | 5–9 days |
| Birkenhead | England | 5–9 days |